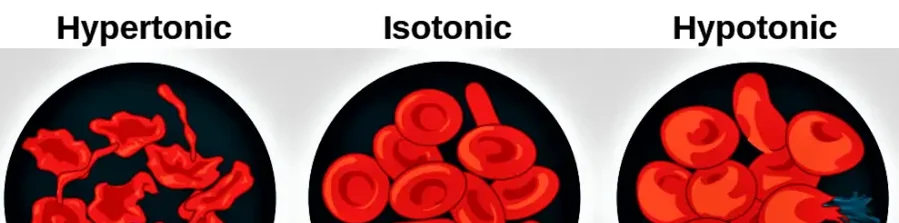
- Methods of adjusting isotonicity of a solution is essential in pharmaceutical and medical applications to prevent cellular damage or discomfort.
- The goal is to match the osmotic pressure of the solution to physiological fluids, such as blood or tears (~290 mOsmol/kg).
Class I Method (Cryoscopic Method):
Based on Freezing Point Depression:
- Uses the formula:
- $ΔT=K_f×m$
- Where:
- ΔT = change in freezing point
- Kf = cryoscopic constant
- m = molality of solute
- Adjust the molality to achieve the desired change in freezing point, which corresponds to isotonicity.
Class II Method (Osmotic Pressure Method):
Based on Osmotic Pressure:
- Formula:
- $π= i ×M ×R ×T$
- Where:
- π = osmotic pressure
- i = van’t Hoff factor (number of particles)
- M = molarity
- R = ideal gas constant
- T = temperature (Kelvin)
- Adjust solute concentration to match the osmotic pressure of an isotonic solution.
Advertisements
White-Vincent Methods of adjusting isotonicity:
Based on Isotonic Equivalents:
- Formula:
- $IE=w/MW×i$
- Where:
- IE = isotonic equivalent
- w = weight of solute (g)
- MW = molecular weight of solute
- i = van’t Hoff factor
- Calculate isotonic equivalents for all solutes and determine the amount of isotonicity-adjusting substance needed.
Sprowls Methods of adjusting isotonicity:
Based on Equivalent Weights:
- Formula: $EW=M/100×φ$
- Where:
- EW = equivalent weight
- M = molecular weight of solute
- φ = osmotic coefficient
- Determine the total equivalent weight of solutes and adjust accordingly to achieve isotonicity.