Nitroglycerin is a potent anti-anginal drug that relieves chest pain by dilating coronary arteries and reducing myocardial oxygen demand.
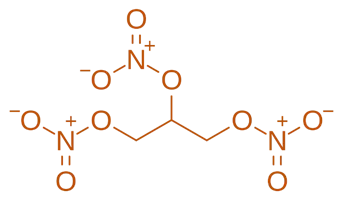
Structure of Nitroglycerin
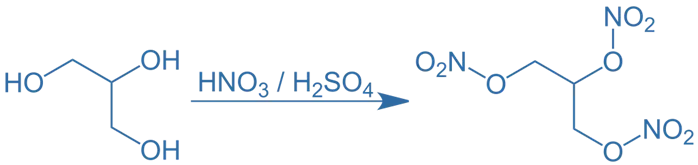
- It is also known as glyceryl trinitrate, is a nitrate ester of glycerol. It contains three nitrate groups esterified to the three hydroxyl groups of glycerol.
- Chemical Formula: C₃H₅N₃O₉
Advertisements
Mode of Action
- Nitric Oxide Release: Nitroglycerin is metabolized to release NO.
- Vasodilation: NO activates guanylate cyclase, increasing cyclic GMP and causing smooth muscle relaxation.
- Reduction of Cardiac Workload: Dilates veins more than arteries, reducing venous return (preload) and myocardial oxygen demand.
Advertisements
Uses
- Angina Pectoris: Acute relief of anginal attacks.
- Heart Failure: Management of acute decompensated heart failure.
- Hypertension: As an adjunct in hypertensive emergencies.
- Anal Fistulas: Applied topically to promote vasodilation and reduce pressure.
Structure-Activity Relationship (SAR)
- Nitrate Ester Groups: Essential for NO release; the number and position of nitrate groups influence potency and duration.
- Glycerol Backbone: Provides flexibility, allowing proper orientation for enzyme-mediated NO release.
- Hydroxyl Substituents: Affect solubility and bioavailability.
Advertisements
Synthesis of Nitroglycerin