A Quantitative Approach to Factors Influencing Solubility of Drugs solubility involves analyzing how physicochemical properties and environmental conditions affect dissolution.
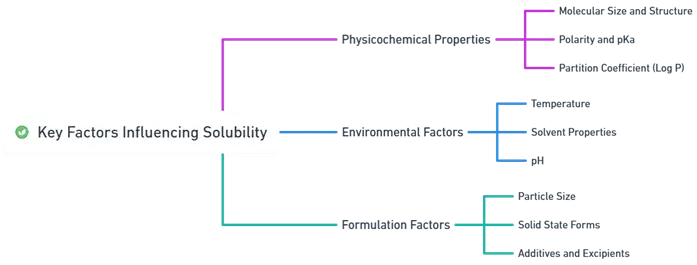
Key Factors Influencing Solubility:
-
Physicochemical Properties:
- Molecular Size and Structure: Larger molecules typically have lower solubility.
- Polarity and pKa: Polar drugs and ionized forms are more soluble in water; the Henderson-Hasselbalch equation determines ionization.
- Partition Coefficient (Log P): Indicates lipophilicity; higher log P means higher solubility in lipophilic solvents.
-
Environmental Factors Influencing Solubility of Drugs:
- Temperature: Solubility generally increases with temperature (van’t Hoff equation).
- Solvent Properties: High dielectric constant solvents (e.g., water) dissolve polar substances better.
- pH: Affects drug ionization and solubility; buffering maintains pH stability.
-
Formulation Factors:
- Particle Size: Smaller particles dissolve faster (Noyes-Whitney equation).
- Solid State Forms: Amorphous forms are more soluble than crystalline ones.
- Additives and Excipients: Surfactants, cyclodextrins, and co-solvents enhance solubility.
Click Here to Watch the Best Pharma Videos