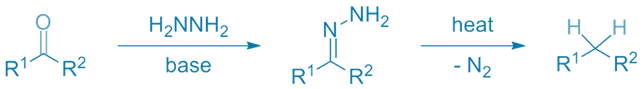
- This article explains about Wolff-Kishner Reduction converts aldehydes and ketones to hydrocarbons under strong base and heat.
- Type: Strong base reduction
Purpose of Wolff-Kishner Reduction:
- Converts aldehydes and ketones to alkanes, like the Clemmensen reduction.
- Basic conditions, so suitable for acid-sensitive compounds.
Advertisements
Reagents:
- Hydrazine (NH₂NH₂)
- Strong base (e.g., KOH)
- Heat
- Often done in high-boiling solvents like ethylene glycol.
Mechanism of Wolff-Kishner Reduction:
-
Step 1: Hydrazone Formation
- Carbonyl compound reacts with hydrazine:
- R₂C=O + H₂NNH₂ → R₂C=NNH₂ + H₂O
- Carbonyl compound reacts with hydrazine:
-
Step 2: Deprotonation
- Under basic conditions, the hydrazone is deprotonated:
- R₂C=NNH₂ + OH⁻ → R₂C=NNH⁻ + H₂O
- Under basic conditions, the hydrazone is deprotonated:
-
Step 3: Resonance and Rearrangement
- Electron rearrangement creates a double bond and leaves a good leaving group:
- R₂C=NNH⁻ → R₂CH⁻ + N₂↑
- Electron rearrangement creates a double bond and leaves a good leaving group:
-
Step 4: Protonation
- Final deprotonation/protonation cycle yields the alkane:
- R₂CH⁻ + H₂O → R₂CH₂ + OH⁻
- Nitrogen gas has evolved — this drives the reaction forward (Le Chatelier’s principle).

- Final deprotonation/protonation cycle yields the alkane:
Advertisements
Example:
Ph-CO-CH₃ + NH₂NH₂/KOH → Ph-CH₂-CH₃ + N₂↑
Key Point:
- Neutralizes the carbonyl group fully to CH₂.
- Works where acidic conditions of Clemmensen would fail.
Advertisements