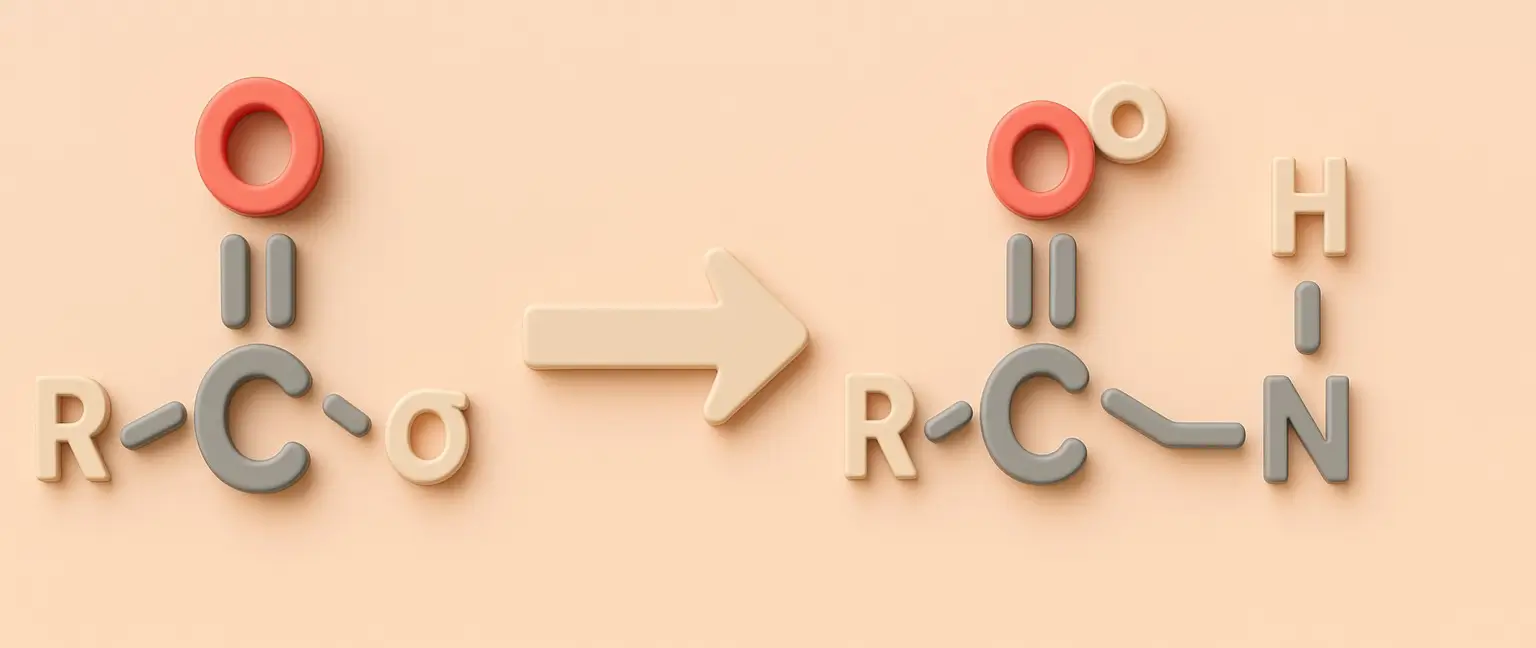Chemical reaction of Carbonyl compounds Definition
- Chemical reaction of Carbonyl compounds refers to the set of organic reactions involving the nucleophilic addition or substitution at the carbonyl carbon of aldehydes and ketones, often driven by the electrophilic nature of the carbon in the C=O bond.
1. Nucleophilic Addition Reactions of Carbonyl compounds:
-
Addition of Hydrogen Cyanide (HCN):
- Aldehydes and ketones react with HCN to form cyanohydrins.
- Example: Acetone + HCN → Acetone cyanohydrin (CH₃COHCN).
-
Addition of Alcohols (Hemiacetal and Acetal Formation):
-
Addition of Amines (Imine Formation):
- Reaction with primary amines (RNH₂) yields imines (Schiff bases).
- Example: Acetone + Methylamine → Imine.
2. Oxidation Reactions of Carbonyl compounds:
-
Oxidation to Carboxylic Acids:
- Aldehydes are easily oxidized to carboxylic acids using oxidizing agents like KMnO₄, K₂Cr₂O₇, or Tollens’ reagent.
- Example: Ethanal (CH₃CHO) oxidized to acetic acid (CH₃COOH).
-
Mild Oxidation (Tollens’ and Fehling’s Test):
- Tollens’ test: Aldehydes react with Tollens’ reagent (AgNO₃ in ammonia) to form a silver mirror.
- Fehling’s test: Aldehydes reduce Fehling’s solution to a red precipitate of Cu₂O.
3. Reduction Reactions:
-
Reduction to Alcohols:
- Aldehydes and ketones can be reduced to primary and secondary alcohols respectively using reducing agents like NaBH₄ or LiAlH₄.
- Example: Propanone (CH₃COCH₃) reduced to isopropanol (CH₃CHOHCH₃).
-
Catalytic Hydrogenation:
- Reduction using H₂ gas in the presence of a catalyst (e.g., Pt, Pd) to form alcohols.
4. Aldol Condensation:
- Aldehydes and ketones containing α-hydrogens undergo aldol condensation in the presence of a base to form β-hydroxy aldehydes or ketones, which can further dehydrate to α,β-unsaturated carbonyl compounds.
- Example: Two molecules of acetaldehyde react to form 3-hydroxybutanal (aldol) which can dehydrate to crotonaldehyde.
5. Cannizzaro Reaction:
- Aldehydes without α-hydrogens react with a strong base (e.g., NaOH) to produce a mixture of a carboxylic acid and an alcohol.
- Example: Formaldehyde (HCHO) in NaOH → methanol (CH₃OH) + formic acid (HCOOH).
6. Grignard Reaction:
- Aldehydes and ketones react with Grignard reagents (RMgX) to form alcohols.
- Example: Acetone + Methylmagnesium bromide → 2-Methyl-2-propanol.
7. Haloform Reaction:
- Methyl ketones (CH₃COR) react with halogens in the presence of a base to produce a haloform (CHX₃, where X is a halogen) and a carboxylate ion.
- Example: Acetone + I₂ + NaOH → Iodoform (CHI₃) + Sodium acetate (CH₃COONa).
Click Here to Watch the Best Pharma Videos