Claisen-Schmidt Condensation forms α,β-unsaturated carbonyl compounds by reacting aromatic aldehydes with ketones in base.
- The Claisen–Schmidt condensation is a base-catalyzed aldol condensation between an aromatic aldehyde and a ketone (or sometimes another aldehyde) with α-hydrogens, typically resulting in an α,β-unsaturated carbonyl compound.
Advertisements
General Reaction:
Aromatic aldehyde + Aliphatic ketone —(Base/heat) → α,β-unsaturated ketone + H₂O
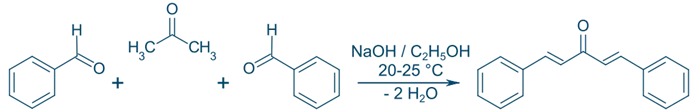
Example of Claisen-Schmidt Condensation:
Benzaldehyde + Acetophenone → Chalcone
Advertisements
Ph–CHO + Ph–COCH₃ → Ph–CH=CH–CO–Ph + H₂O
Advertisements
Reaction Conditions
- Base catalyst: Typically, NaOH or KOH
- Solvent: Ethanol or methanol
- Heat: Often required to drive the elimination step
Mechanism (Step-by-Step)
Let’s take benzaldehyde and acetophenone as an example:
-
Step 1: Enolate Formation
- The base abstracts an α-proton from acetophenone, forming an enolate ion.
- Ph–COCH₃ + OH⁻ → Ph–C⁻=CH₂ (enolate) + H₂O
- The base abstracts an α-proton from acetophenone, forming an enolate ion.
-
Step 2: Nucleophilic Addition to Aldehyde
- The enolate ion attacks the carbonyl carbon of benzaldehyde.
- Forms a β-hydroxy ketone intermediate (aldol addition product).
- Ph–C⁻=CH₂ + Ph–CHO → Ph–CH(OH)–CH₂–CO–Ph
-
Step 3: Aldol Condensation (Elimination)
- Under heat, the β-hydroxy ketone eliminates water to form an α,β-unsaturated ketone (chalcone).
- Ph–CH(OH)–CH₂–CO–Ph → Ph–CH=CH–CO–Ph + H₂O
- This elimination proceeds via an E1cb mechanism (conjugate base elimination), where:
- The base removes a proton from the β-carbon.
- The resulting enolate eliminates the hydroxide (or water) from the α-carbon.
- Under heat, the β-hydroxy ketone eliminates water to form an α,β-unsaturated ketone (chalcone).
Advertisements
Features of Claisen–Schmidt Condensation
- Crossed aldol: between two different carbonyl compounds (one aldehyde, one ketone).
- One component (aldehyde) usually lacks α-hydrogens to prevent self-condensation.
- Forms conjugated systems: the α,β-unsaturated carbonyl is stabilized by resonance.
- Used in synthesis of:
- Chalcones
- Flavonoids
- Pharmaceuticals
- Natural products
Key Considerations of Claisen-Schmidt Condensation
- Selectivity: Controlled by using one reactant without α-hydrogens.
- Base choice: Strong enough to form enolate but not so strong to cause side reactions.
- Solvent: Must solubilize both organic and inorganic components.
- Temperature: Higher temperatures favor condensation (dehydration step).
Click Here to Watch the Best Pharma Videos
Advertisements