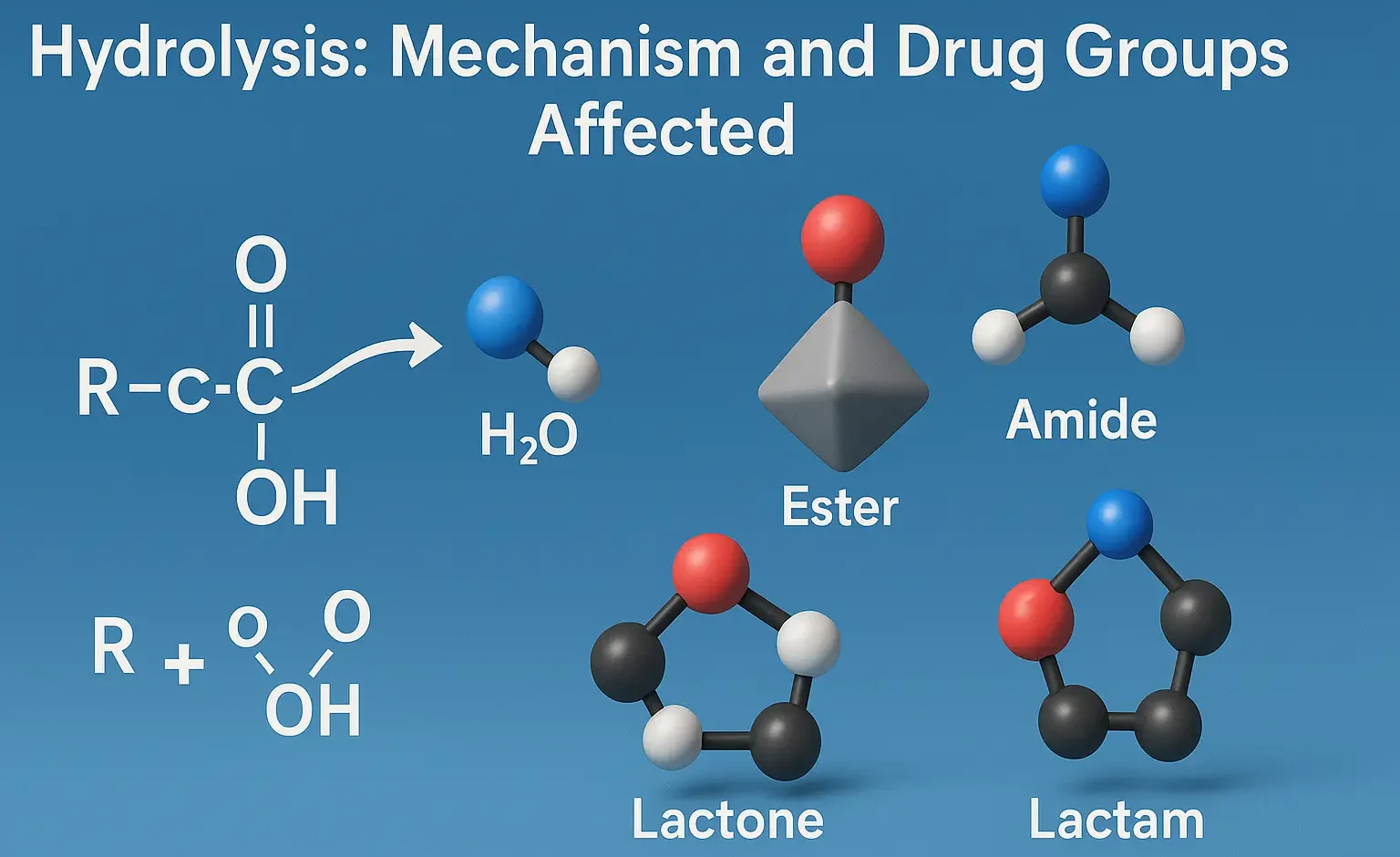
Mechanism of Hydrolysis:
- Hydrolysis involves nucleophilic attack of water on labile functional groups, breaking chemical bonds such as esters and amides.
- Example:
$\mathrm{R{-}COOR’} + H_{2}O \;\longrightarrow\; \mathrm{R{-}COOH} + \mathrm{R’OH}$
Advertisements
Commonly Affected Drug Classes:
- Esters: aspirin, procaine
- Amides: lidocaine, procainamide
- Lactams: penicillins, cephalosporins
- Carbamates, imines
Stabilization Strategies for Hydrolysis
| Strategy | Explanation |
| pH control (buffering) | Adjust formulation to pH where drug is most stable (minimizes specific acid/base catalysis) |
| Use of non-aqueous solvents | Reduce water content by using ethanol, propylene glycol, or glycerol |
| Dry formulations | Prefer solid dosage forms like tablets or powders to avoid water |
| Lyophilization (freeze drying) | Remove water from the formulation; reconstituted only before use |
| Use of surfactants/micelles | Protect labile groups through encapsulation |
| Complex formation | Use stabilizing agents (e.g., cyclodextrins) to form protective complexes |
| Packaging in moisture-resistant containers | Use desiccants, foil blister packs, and sealed containers to avoid moisture |
Click Here to Watch the Best Pharma Videos
Advertisements
Advertisements