Understanding the States of Matter and Their Properties
States of matter refer to the distinct forms that different phases of matter take based on the arrangement and energy of their particles. The three primary states of matter are solid, liquid, and gas. Each state has unique characteristics and behaviors that define how substances interact with their environment.
Primary States of Matter
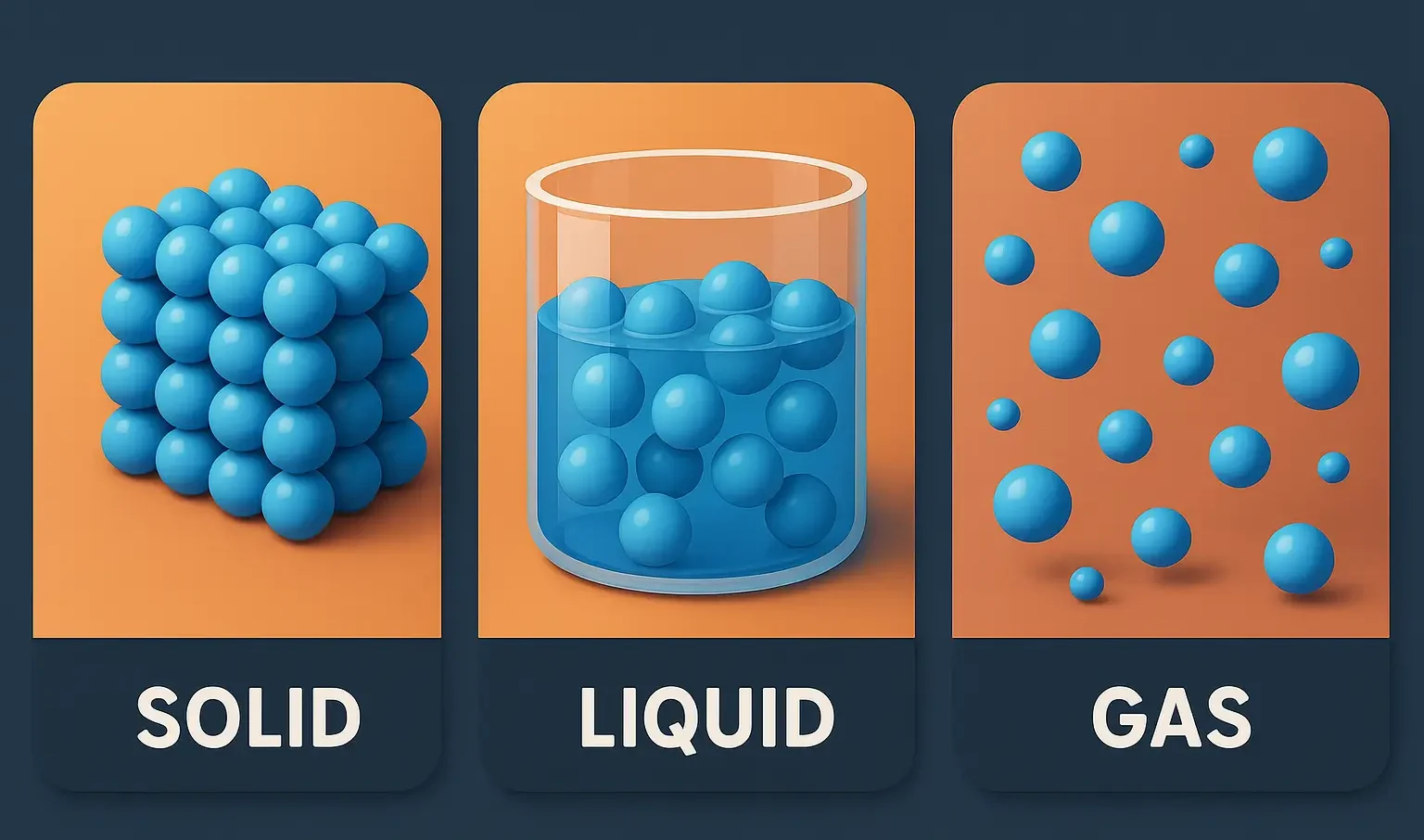
1. Solid
- Characteristics: Solids have a definite shape and volume. Their particles are tightly packed in a fixed, orderly structure.
- Particle Movement: Particles vibrate in place but do not move freely.
- Examples: Ice, wood, and metals like iron.
2. Liquid
- Characteristics: Liquids maintain a fixed volume but take the shape of their container. Particles are close together but not in a fixed position.
- Particle Movement: Particles slide past one another, allowing the liquid to flow.
- Examples: Water, oil, and mercury.
3. Gas
- Characteristics: Gases have neither a fixed shape nor volume. Their particles are widely spaced and move independently.
- Particle Movement: Particles move rapidly and freely in all directions.
- Examples: Oxygen, nitrogen, and carbon dioxide.
Key Properties of Matter
The properties of matter help identify and classify substances and are divided into physical and chemical properties.
-
Physical Properties
- These can be observed without altering the substance’s identity:
- Density: Mass per unit volume (e.g., water = 1 g/cm³)
- Color: Visual appearance (e.g., gold is yellow)
- Melting Point: Solid to liquid transition temperature (e.g., ice at 0°C)
- Boiling Point: Liquid to gas transition (e.g., water at 100°C)
- Solubility: Ability to dissolve in a solvent (e.g., salt in water)
- These can be observed without altering the substance’s identity:
-
Chemical Properties
- These involve a change in substance composition:
- Reactivity: Tendency to undergo chemical change (e.g., sodium reacts with water)
- Flammability: Capability to burn (e.g., gasoline)
- Oxidation States, Acidity/Basicity, and Toxicity are other notable examples.
- These involve a change in substance composition:
Understanding the states of matter and their properties is crucial in science and everyday life, from cooking and freezing to chemical reactions and industrial processes.
Thank you for reading from Firsthope's notes, don't forget to check YouTube videos!