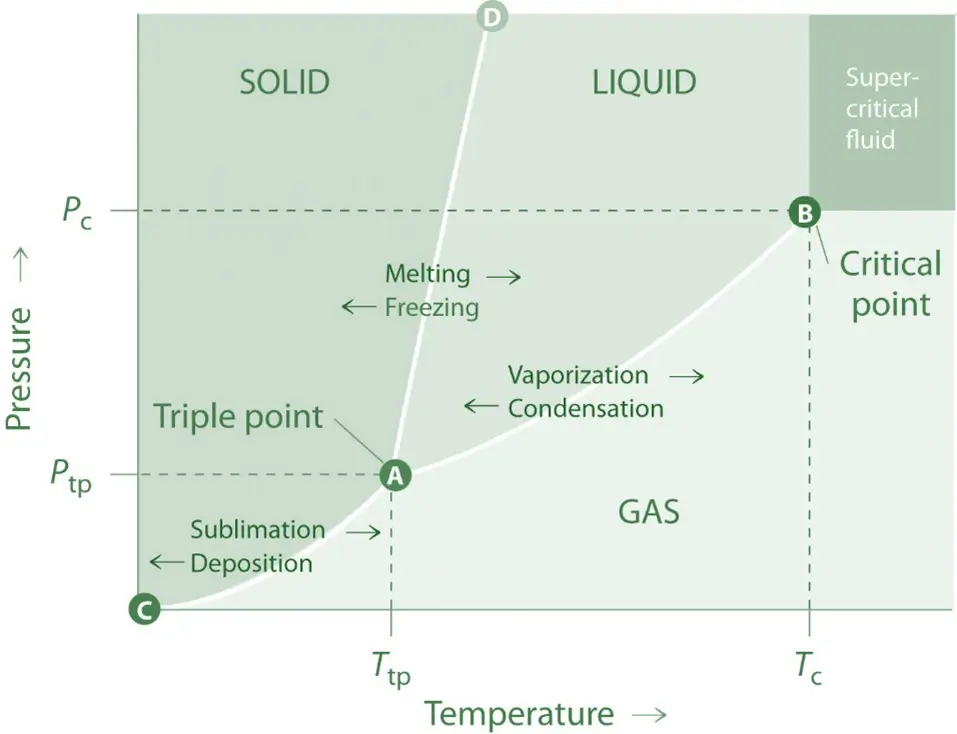
Definition of Sublimation Critical Point:
- The specific temperature and pressure where a substance can exist simultaneously in solid, liquid, and gas phases.
Importance of Sublimation:
- Helps understand sublimation, where a substance transitions directly from solid to gas below the triple point.
Example of Sublimation Critical Point:
- Carbon dioxide sublimates at -78.5°C under standard atmospheric pressure but can exist as a liquid at higher pressures.
Graph Explanation:
- A phase diagram typically shows the temperature and pressure relationship, with regions indicating solid, liquid, and gas phases.
- The sublimation curve shows the boundary where a solid can directly become a gas.
- At the critical point, this curve intersects the critical point for gas and liquid phases.
Advertisements
Importance:
- Industrial Processes: Supercritical fluids are used in extraction (e.g., supercritical CO₂ for decaffeinating coffee) and as solvents in chemical reactions.
- Material Science: Understanding the critical point is essential for designing equipment that handles substances near their critical conditions.
- Enhanced Oil Recovery: Utilizes supercritical fluids to extract oil from reservoirs.

